Total synthesis and stereochemical revision of relgro and 10′-oxorelgro†
Abstract
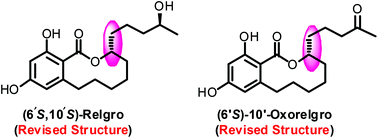
The first asymmetric total synthesis and stereochemical assignments of 10-membered macrolactones relgro and 10′-oxorelgro are disclosed. To this end, palladium-catalyzed Stille coupling, the Mitsunobu reaction, ring-closing metathesis, EDCI promoted coupling and the Jacobsen hydrolytic kinetic resolution are used as key steps. The total synthesis followed by thorough evaluation of the optical rotation and CD spectral data led to the revision of the absolute configuration at C-6′ for both relgro and 10′-oxorelgro. Moreover, the 1H as well as 13C NMR data are reported for the first time for relgro.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...