Tight-binding inhibition of jack bean α-mannosidase by glycoimidazole clusters†
Abstract
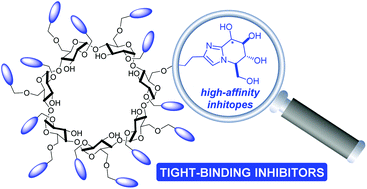
The best multivalent effects observed in glycosidase inhibition have been achieved so far with jack bean α-mannosidase (JBα-man) using iminosugar clusters based on weakly binding mismatching active-site-directed inhibiting epitopes (inhitopes) in the D-gluco series. Here, we synthesize and evaluate as JBα-man inhibitors a series of mono- to 14-valent glycoimidazoles with inhitopes displaying inhibition values up to the range of hundreds of nMs to study the impact of inhitope affinity on the multivalent effect. The most potent inhibitor of the series, a 14-valent mannoimidazole derivative, inhibits JBα-man with a nanomolar Ki value (2 ± 0.5 nM) and binding enhancements observed are, at best, relatively small (up to 25-fold on a valency-corrected basis). The results of this study support the fact that JBα-man-inhitope affinity and the strength of the inhibitory multivalent effect evolve in the opposite direction. The major impact of the glycoimidazole-based inhitope is found on the binding scenario; most of the synthesized mannoimidazole clusters as well as a 14-valent glucoimidazole derivative prove to be tight binding inhibitors of JBα-man.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...