Manganese(iii) acetate-mediated direct C(sp2)–H-sulfonylation of enamides with sodium and lithium sulfinates†
Abstract
A Mn(OAc)3 mediated oxidative C(sp2)–H sulfonylation of enamides and encarbamates with sodium and lithium sulfinates is reported. This operationally simple transformation provides a straightforward and highly stereoselective access to (E)-β-amidovinyl sulfones in moderate to excellent yields. The reaction proceeds readily under mild conditions at room temperature and tolerates various sensitive functional groups. This process affords exclusively (E)-configurated β-amidovinyl sulfones independent of the starting material configuration. Moreover, a direct transformation of organolithium reagents and sulfur dioxide into β-amidovinyl sulfones is described.
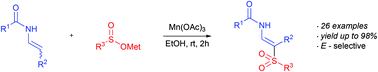
- This article is part of the themed collections: Synthetic methodology in OBC and Direct C-H Functionalization in Method Development and Late Stage Functionalization


 Please wait while we load your content...
Please wait while we load your content...