Structural flexibility versus rigidity of the aromatic unit of DNA ligands: binding of aza- and azoniastilbene derivatives to duplex and quadruplex DNA†
Abstract
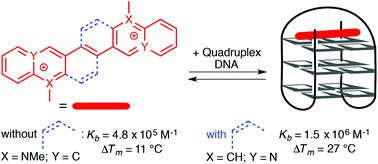
The known azastilbene (E)-1,2-di(quinolin-3-yl)ethane (2a) and the novel azoniastilbene derivatives (E)-2-(2-(naphthalen-2-yl)vinyl)quinolizinium (2b) and (E)-3,3′-(ethane-1,2-diyl)bis(1-methylquinolinin-1-ium) (2c) were synthesized. Their interactions with duplex and quadruplex DNA (G4-DNA) were studied by photometric, fluorimetric, polarimetric and flow-LD analysis, and by thermal DNA denaturation studies, as well as by 1H-NMR spectroscopy. The main goal of this study was a comparison of these conformationally flexible compounds with the known G4-DNA-binding diazoniadibenzo[b,k]chrysenes, that have a comparable π-system extent, but a rigid structure. We have observed that the aza- and azoniastilbene derivatives 2a–c, i.e. compounds with almost the same spatial dimensions and steric demand, bind to DNA with an affinity and selectivity that depends significantly on the number of positive charges. Whereas the charge neutral derivative 2a binds unspecifically to the DNA backbone of duplex DNA, the ionic compounds 2b and 2c are typical DNA intercalators. Notably, the bis-quinolinium derivative 2c binds to G4-DNA with moderate affinity (Kb = 4.8 × 105 M−1) and also stabilizes the G4-DNA towards thermal denaturation (ΔTm = 11 °C at ligand–DNA ratio = 5.0). Strikingly, the corresponding rigid counterpart, 4a,12a-diazonia-8,16-dimethyldibenzo[b,k]chrysene, stabilizes the G4-DNA to an even greater extent under identical conditions (ΔTm = 27 °C). These results indicate that the increased flexibility of a G4-DNA ligand does not necessarily lead to stronger interactions with the G4-DNA as compared with rigid ligands that have essentially the same size and π system extent.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...