A gold-catalyzed facile intramolecular rearrangement and cyclization sequence for synthesis of 2,5-dihydrofurans†
Abstract
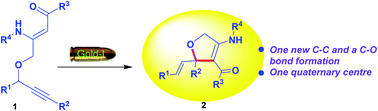
An efficient gold-catalyzed intramolecular rearrangement and cyclization protocol was developed for synthesis of 2,5-dihydrofuran derivatives from O-propargyl β-enaminones. In this organic transformation new C–C and C–O bonds are formed under mild reaction conditions; this includes the formation of a quaternary centre.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...