Evaluating aryl esters as bench-stable C(1)-ammonium enolate precursors in catalytic, enantioselective Michael addition–lactonisations†
Abstract
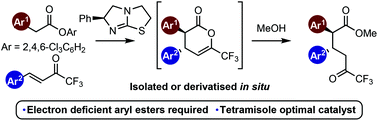
An evaluation of a range of aryl, alkyl and vinyl esters as prospective C(1)-ammonium enolate precursors in enantioselective Michael addition–lactonisation processes with (E)-trifluoromethylenones using isothiourea catalysis is reported. Electron deficient aryl esters are required for reactivity, with 2,4,6-trichlorophenyl esters providing optimal product yields. Catalyst screening showed that tetramisole was the most effective isothiourea catalyst, giving the desired dihydropyranone product in excellent yield and stereoselectivity (up to 90 : 10 dr and 98 : 2 er). The scope and limitations of this process have been evaluated, with a range of diester products being generated after ring-opening with MeOH to give stereodefined dihydropyranones with excellent stereocontrol (10 examples, typically ∼90 : 10 dr and >95 : 5 er).
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...