pH-Regulated anion transport activities of bis(iminourea) derivatives across the cell and vesicle membrane†
Abstract
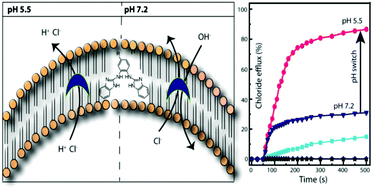
Recently, synthetic anion transporters have gained considerable attention because of their ability to disrupt cellular anion homeostasis and promote cell death. Herein, we report the development of bis(iminourea) derivatives as a new class of selective Cl− ion carrier. The bis(iminourea) derivatives were synthesized via a one-pot approach under mild reaction conditions. The presence of iminourea moieties suggests that the bis(iminourea) derivatives can be considered as unique guanidine mimics, indicating that the protonated framework could have much stronger anion recognition properties. The cooperative interactions of H+ and Cl− ions with these iminourea moieties results in the efficient transport of HCl across the lipid bilayer in an acidic environment. Under physiological conditions these compounds weakly transport Cl− ions via an antiport exchange mechanism. This pH-dependent gating/switching behavior (9-fold) within a narrow window could be due to the apparent pKa values (6.2–6.7) of the compounds within the lipid bilayer. The disruption of ionic homeostasis by the potent compounds was found to induce cell death.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...