TiCl4-n-Bu3N-mediated cascade annulation of ketones with α-ketoesters: a facile synthesis of highly substituted fused γ-alkylidene-butenolides†
Abstract
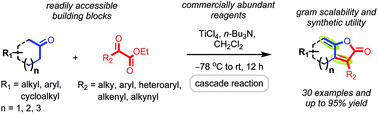
A facile protocol for the synthesis of highly substituted fused γ-alkylidene butenolides using direct annulation of ketones with α-ketoesters, which proceeds through TiCl4-n-Bu3N mediated aldol addition followed by an intramolecular enol-lactonization/cyclization cascade, is reported. Diverse 6-5, 7-5 and 8-5 fused bicyclic γ-ylidene butenolides and highly substituted monocyclic analogs related to biologically relevant natural products were prepared from readily accessible ketone and α-ketoester building blocks. The highly step-economic cascade nature, good substrate scope, easy access to complex products with good to excellent yields, gram-scalability, demonstration of synthetic utility, and unambiguous structural confirmation through X-ray crystallography analyses and analogy are the salient features of this work.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...