Rhodium(iii)-catalyzed chemoselective C–H functionalization of benzamides with methyleneoxetanones controlled by the solvent†
Abstract
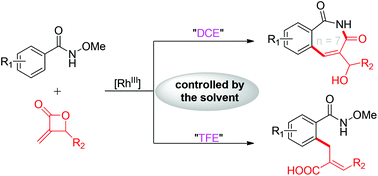
Described herein is a Rh(III)-catalyzed and solvent-controlled double C–H functionalization of common benzamides via selective acyl C–O cleavage (β-H elimination) or alkyl C–O cleavage (β-O elimination) of the methyleneoxetanone substrate, which provides a straightforward way for the divergent synthesis of chain alkylated benzamides and seven-membered 1H-benzo[c]azepine-1,3(2H)-diones in a highly chemoselective manner. Through a series of experimental investigations together with theoretical studies, the effect of the solvent has been systematically elucidated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...