Decarboxylative acylation of N-free indoles enabled by a catalytic amount of copper catalyst and liquid-assisted grinding†
Abstract
A facile decarboxylative acylation of N-free indoles with α-ketonates via liquid-assisted grinding was reported. The reaction requires only a catalytic amount of Cu(OAc)2·H2O in combination with O2 as the terminal oxidant to give various 3-acylindoles with high efficiency. Additionally, this new methodology was applicable to a gram-scale synthesis.
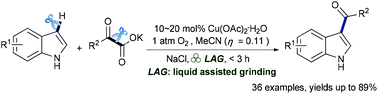
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...