Synthesis of benzoxazoles via the copper-catalyzed hydroamination of alkynones with 2-aminophenols†
Abstract
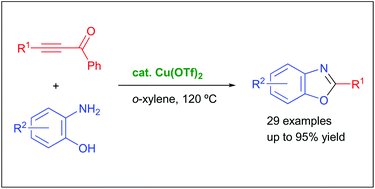
We describe herein the synthetic method to benzoxazole derivatives via the copper-catalyzed hydroamination of alkynones with 2-aminophenols. The method produced a wide variety of functionalized benzoxazole derivatives in good yields. Preliminary mechanistic experiments revealed that the reaction would proceed through the copper-catalyzed hydroamination of alkynones and the sequential intramolecular cyclization of β-iminoketones/elimination of acetophenone promoted by the copper catalyst.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...