Cytochrome c–poly(acrylic acid) conjugates with improved peroxidase turnover number†
Abstract
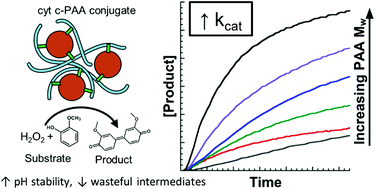
Cytochrome c–poly(acrylic acid) (cyt c–PAA) conjugates with 34-fold enchancement in peroxidase turnover number (kcat) are reported. Cyt c–PAA conjugates were prepared by carbodiimide coupling. PAA with molecular weight (Mw) ranging from 1.8k to 250k g mol−1 were employed, and the effect of PAA Mw on peroxiodase kinetics was assessed. The kcat value increased with increased Mw of PAA, ranging from 0.077(±0.002) s−1 in the absence of PAA to 2.66(±0.08) s−1 for the conjugate of cyt c with 250k PAA. Enzymatic activity studies over pH 6–8 indicated improved activity for cyt c–PAA conjugates at neutral or slightly alkaline pH. Examination of the cyt c heme spectroscopy in the presence of H2O2 revealed that formation of compound III, a reactive intermediate that leads to enzyme inactivation, was supressed in cyt c–PAA conjugates. Thus, we suggest the kcat enhancement can be attributed to acidification of the pH microenvironment and inhibition of the formation of a reactive intermediate that deactivates cyt c during the catalytic cycle.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...
