Unprecedented E-stereoselectivity on the sigmatropic Hurd–Claisen rearrangement of Morita–Baylis–Hillman adducts: a joint experimental–theoretical study†
Abstract
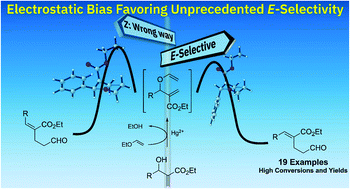
Herein we report the first systematic investigation of the tandem mercury(II) catalysed transvinylation/Hurd–Claisen rearrangement of MBH adducts derived from alkyl acrylates. This is the first report of E-selectivity for MBH adducts with alkyl side chains and is complementary to the previously reported Johnson–Claisen and Eschenmoser–Claisen rearrangements. The rearrangement products were obtained in good yields and could be readily converted to 2-alkenyl δ-valerolactones. Combined DFT and F-SAPT studies demonstrate that reaction rates are primarily governed by non-covalent interactions dictating the relative stability of the transition states. Our F-SAPT calculations revealed that the hyperconjugative effects are not so significant, but that electrostatic interactions, instead, are the driving forces for the relative E : Z stereoselectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...