The one-pot nonhydrolysis Staudinger reaction and Staudinger or SPAAC ligation†
Abstract
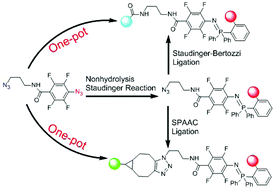
The combined usage of two bioorthogonal reactions can provide hetero-bifunctional molecules under physiological conditions for various applications. Based on the Nonhydrolysis Staudinger Reaction (NSR), we design and develop a bisazido linker 1 for chemoselective dual-functionalization without the need of protection using catalyst-free and one-pot procedures. The NSR is much faster with tetrafluorinated aromatic azide than that the Staudinger–Bertozzi or SPAAC ligation with alkyl azide, as revealed by HPLC analysis and fluorescence kinetics. Based on the tandem NSR and Staudinger–Bertozzi ligation, we prepare a molecular beacon 7 from 1 in one-pot synthesis with a recovery yield of 32%. When a faster SPAAC ligation is used instead of the Staudinger–Bertozzi ligation, compound 8 is prepared in the tandem NSR and SPAAC reactions with a recovery yield of 59%. As a proof-of-concept study, the tandem NSR and SPAAC ligation is further used to produce a FRET-based dyad in living cells, as revealed by dual-color bioimaging. This work shows that NSR can be combined with other bioorthogonal reactions without the need of protection in one-pot.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...