Metal- and radical-free aerobic oxidation of heteroaromatic methanes: an efficient synthesis of heteroaromatic aldehydes†
Abstract
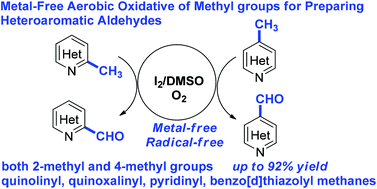
A metal-free and radical-free synthesis of heteroaromatic aldehydes was developed through aerobic oxidation of methyl groups in an I2/DMSO/O2 catalytic system. Under the reaction conditions, various functional groups such as methoxy, aldehyde, ester, nitro, amide, and halo (F, Cl, Br) groups were well tolerated. The bioactive compounds like chlorchinaldin derivative and papaverine were also oxidized to the corresponding aldehydes and ketones. This reaction provided an efficient method for preparing the valuable heteroaromatic aldehydes.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...