A divergent strategy to synthesize gabosines featuring a switchable two-way aldol cyclization†
Abstract
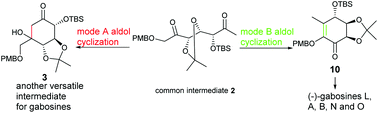
Gabosines and their natural analogues, belonging to C7 carbasugars, have attracted great attention in synthesis due to their rich structural variety and promising biological activities. A new diversity-oriented approach for the gabosine-type carbasugars based on a tunable regioselective aldol cyclization of flexible precursor 2 is explored. Two cyclization modes (A and B) of the precursor can be well controlled by switching promoters to selectively produce two resulting cyclohexa(e)nones 3 and 10, both of which are versatile intermediates for various C7 carbasugars. After the conversion of 3 to eight natural carbasugars, the utility of intermediate 10 is illustrated by the first synthesis of (−)-gabosine L, as well as the new synthesis of (−)-gabosine A, (−)-gabosine B, (−)-gabosine N and (−)-gabosine O. The chemical structure and the absolute configuration of (−)-gabosine L are confirmed by its total synthesis.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...