Pd-Catalyzed directed CH-(hetero)arylation of cyclic α-amino acids: effects of substituents and the ring size†
Abstract
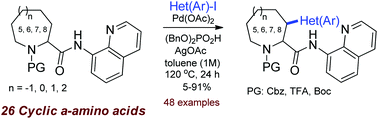
A systematic study on the directed Pd-catalyzed (hetero)arylation of 26 substituted cyclic α-amino acids at the C(3)-atom was performed. For the first time, the 7- and 8-membered cyclic amino acids were introduced to C–H activation. 8-Aminoquinoline was used as a directing group. Effects of the ring size and the substituents on the reaction efficacy and stereoselectivity were studied.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...