Substituent-controlled racemization of dissymmetric coordination capsules†
Abstract
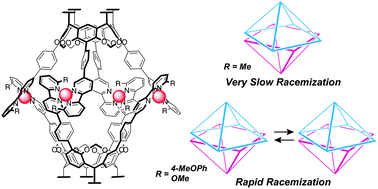
We report the effect of substituents (methyl, isopropyl, methoxy, and methoxyphenyl) at the 6′-position of the 2,2′-bipyridyl arms on the racemization of dissymmetric coordination capsules 1a–d. When the capsules included (R)-4,4′-diacetoxy-2,2′-benzyloxycarboxyl-biphenyl ((R)-3), the (M)-helical conformer was enriched with a diastereomeric excess (de%) of >98% for 1a, 31% for 1b, 81% for 1c and 75% for 1d. The entrapped guests in 1a, 1c and 1d can be removed by washing the solid containing the host–guest complexes with diethyl ether. The rate of racemization in THF follows the order of 1c > 1d ≫ 1a. X-ray crystal structural analysis and density functional theory calculation of model complex 4c indicate a distorted tetrahedral coordination of the Cu(I) center, and UV-vis absorption spectroscopy indicates similar coordination environments in 1c and 4c. A series of experiments demonstrates that the racemization rate depends on the dihedral angles of the bipyridyl arms, and the angles are regulated by the substituents. The methoxy and methoxyphenyl substituents in 1c and 1d enlarge the dihedral angles of the bipyridyl arms. This facilitates the access of solvent molecules to the Cu(I) centers and promotes racemization. The slower racemization of 1d can be ascribed to the steric protection of the Cu(I) centers from incoming solvent molecules by the p-methoxyphenyl group.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...