Stereoselective functionalization of platensimycin and platencin by sulfa-Michael/aldol reactions†
Abstract
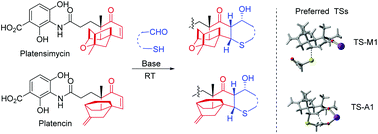
Bioinspired sulfa-Michael/aldol cascade reactions have been developed for the semisynthesis of sulfur-containing heterocyclic derivatives of platensimycin and platencin, with three newly formed contiguous stereogenic centers. Density functional theory calculations revealed the mechanism for the stereochemistry control. This method was used in a synthesis of a platensimycin thiophene analogue with potent antibacterial activities against Staphylococcus aureus.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...