Oxidative oxy-cyclization of 2-alkynylbenzamide enabled by TBAB/Oxone: switchable synthesis of isocoumarin-1-imines and isobenzofuran-1-imine†
Abstract
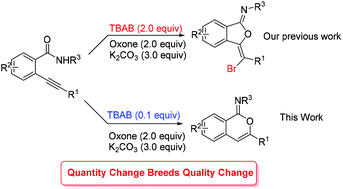
A TBAB-catalyzed oxidative 6-endo-dig oxy-cyclization of 2-alkynylbenzamide is described herein for the synthesis of isocoumarin-1-imines. The transformation proceeds regioselectively and provides the final products with high efficiency and a broad reaction scope. Interestingly, an array of isobenzofuran-1-imines is also achieved under standard conditions when N-phenyl 2-trimethylsilylethynylbenzamides are used as substrates. Mechanism studies show that 3-bromomethenisobenzofuran-1-imine is a pivotal intermediate, which goes through C–O bond migration and debromination to offer the final isocoumarin-1-imines.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...