Iodine-mediated oxidative N–N coupling of secondary amines to hydrazines†
Abstract
An I2-mediated N–N coupling reaction has been established for oxidative dimerization of N-aryl aminopyridines to a variety of novel hydrazine derivatives under mild conditions. This synthetic method does not require use of transition metals and can be conveniently carried out on a gram scale. It is also applicable to diphenylamine and N-alkyl aniline substrates.
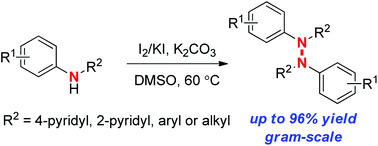
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...