Late-stage C–H amination of abietane diterpenoids†
Abstract
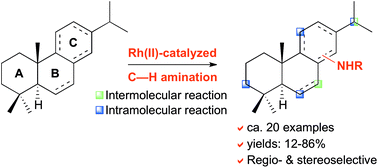
This study aims at highlighting the synthetic versatility of the rhodium-catalyzed C–H amination reactions using iodine(III) oxidants for the late-stage functionalization of natural products. Inter- and intramolecular nitrene insertions have been performed from various abietane diterpenoids, leading to the amination of the C-3, C-6, C-7, C-11 and C-15 positions. Ca. 20 aminated compounds have been isolated with yields of up to 86% and high levels of regio-, chemo- and stereoselectivities.
- This article is part of the themed collections: Synthetic methodology in OBC and Direct C-H Functionalization in Method Development and Late Stage Functionalization


 Please wait while we load your content...
Please wait while we load your content...