Glycoconjugate synthesis using chemoselective ligation†
Abstract
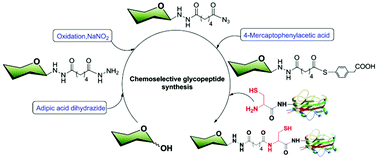
Chemoselective ligation of carbohydrates and polypeptides was achieved using an adipic acid dihydrazide cross-linker. The reducing end of a carbohydrate is efficiently attached to peptides in two steps, constructing a glycoconjugate in high yield and with high regioselectivity, enabling the production of homogeneous glycoconjugates.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...