6-Polyamino-substituted quinolines: synthesis and multiple metal (CuII, HgII and ZnII) monitoring in aqueous media†
Abstract
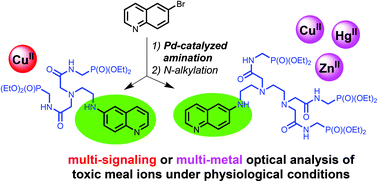
Chemoselective palladium-catalyzed arylation of polyamines with 6-bromoquinoline has been explored to prepare chelators for the detection of metal cations in aqueous media. The introduction of a single aromatic moiety into non-protected polyamine molecules was achieved using the commercially available Pd(dba)2/BINAP precatalyst to afford nitrogen chelators, in which the aromatic signalling unit is directly attached to the polyamine residue. Water-soluble receptors were then synthesized using N-alkylation of these polyamines by hydrophilic coordinating residues. By combining rich photophysical properties of the 6-aminoquinoline unit with a high coordination affinity of chelating polyamines and a hydrophilic character of carboxamido-substituted phosphonic acid diesters in a single molecular device, we synthesized chemosensor 5 for selective double-channel (UV–vis and fluorescence spectroscopies) detection of CuII ions in aqueous media at physiological levels. This receptor is suitable for the analysis of drinking water and fabrication of paper test strips for the naked-eye detection of CuII ions under UV-light. By increasing the number of donor sites we also obtained chemosensor 6 which is efficient for the detection of HgII ions. Moreover, chemosensor 6 is also suitable for multiple detection of metal ions because it chelates not only HgII but also CuII and ZnII ions displaying different responses of emission in the presence of these three cations.


 Please wait while we load your content...
Please wait while we load your content...