Cold-induced aldimine bond cleavage by Tris in Bacillus subtilis alanine racemase†
Abstract
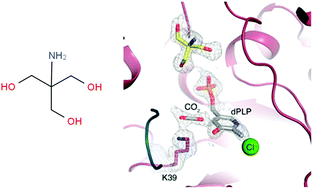
Pyridoxal 5′-phosphate (PLP) is a versatile cofactor involved in a large variety of enzymatic processes. Most of PLP-catalysed reactions, such as those of alanine racemases (AlaRs), present a common resting state in which the PLP is covalently bound to an active-site lysine to form an internal aldimine. The crystal structure of BsAlaR grown in the presence of Tris lacks this covalent linkage and the PLP cofactor appears deformylated. However, loss of activity in a Tris buffer only occurred after the solution was frozen prior to carrying out the enzymatic assay. This evidence strongly suggests that Tris can access the active site at subzero temperatures and behave as an alternate racemase substrate leading to mechanism-based enzyme inactivation, a hypothesis that is supported by additional X-ray structures and theoretical results from QM/MM calculations. Taken together, our findings highlight a possibly underappreciated role for a common buffer component widely used in biochemical and biophysical experiments.


 Please wait while we load your content...
Please wait while we load your content...