N-Heterocyclic carbene-catalyzed diastereoselective synthesis of sulfenylated indanes via sulfa-Michael–Michael (aldol) cascade reactions†
Abstract
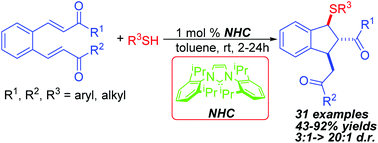
N-Heterocyclic carbene (NHC)-catalyzed diastereoselective synthesis of multisubstituted sulfenylated indanes has been developed. In the presence of 1 mol% NHC, various thiols underwent the sulfa-Michael–Michael cascade reaction with benzenedi(enones) efficiently to form the carbon–sulfur bond and construct sulfenylated indanes in good to excellent yields with high diastereoselectivity. In addition, the NHC-catalyzed sulfa-Michael-aldol cascade reaction between o-formyl chalcone and thiols has also been demonstrated to afford sulfenylated indanes with a free hydroxyl group in moderate yields and good diastereoselectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...