Cp*Co(iii)-catalyzed annulation of azines by C–H/N–N bond activation for the synthesis of isoquinolines†
Abstract
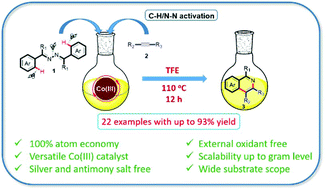
Herein, an efficient, atom economic and external oxidant free approach has been disclosed for the synthesis of isoquinolines. Azines were employed for annulation reactions with alkynes via sequential C–H/N–N bond activation using an air-stable cobalt catalyst. The method takes advantage of the incorporation of both the nitrogen atoms of azines into the desired isoquinoline products, offering the highest atom economy. In addition, the developed protocol works under external oxidant as well as silver salt free conditions. Furthermore, the established methodology features a relatively broad substrate scope with high product yields and scalability up to the gram level.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...