Selective synthesis of spirooxindoles via a two-step reaction of N-phenacylpyridinium bromide, 1,3-indanedione and N-alkylisations†
Abstract
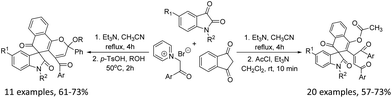
The three-component reaction of 1,3-indanedione and N-alkylisatins with two molecular N-phenacylpyridinium salts in dry acetonitrile in the presence of triethylamine resulted in unique functionalized spiro[indoline-3,4′-naphtho[1,2-b]furan] derivatives in good yields, which were successfully converted to spiro[indoline-3,2′-naphthalen]-4′-yl acetate derivatives by acylation with acetyl chloride in methylene dichloride and alkoxy-substituted spiro[benzo[h]chromene-5,3′-indolines] by acid-catalyzed etherification reaction in alcohol. The reaction mechanism involved the sequential cycloaddition of the in situ-generated pyridinium ylide to dipolarophilic enone, ring-opening of 1,3-indanedione, and selective annulation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...