Programmed synthesis of triarylnitroimidazoles via sequential cross-coupling reactions†
Abstract
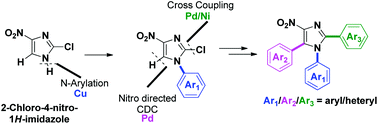
Transition-metal-catalyzed programmed sequential arylation reactions of 2-chloro-4-nitro-1H-imidazoles were achieved. The methods are general and were applied in a chemoselective manner for the synthesis of different multiarylated 4-nitroimidazoles bearing three different aryl groups. A salient feature is Pd-catalyzed hetero–hetero coupling at the C5 position through a NO2 directed cross-dehydrogenative coupling (CDC) approach.
- This article is part of the themed collection: Celebrating the RAOBC symposium


 Please wait while we load your content...
Please wait while we load your content...