C31-Selective substitution of cationic N-heteroaromatic groups into a 3-vinylated chlorophyll-a derivative†
Abstract
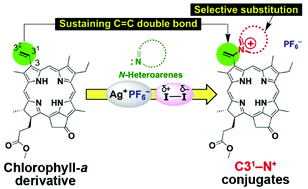
A variety of N-containing heteroarenes were site-selectively introduced at the C31-position of a chlorophyll-a derivative possessing a 3-vinyl group through a C–N+ bond via oxidative reactions using iodine in the presence of a silver(I) salt. Electron-rich N-heteroarenes were effectively substituted, while electron-withdrawing or bulky nitrogen-neighboring substituents suppressed the reactivities. The cationic products formed were characterized by 1D/2D NMR, and their optical properties were also investigated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...