Control of conformation in α-helix mimicking aromatic oligoamide foldamers through interactions between adjacent side-chains†
Abstract
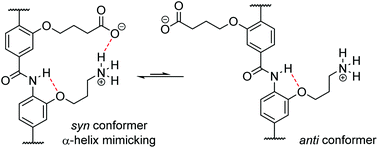
The design, synthesis and structural characterization of non-natural oligomers that adopt well-defined conformations, so called foldamers, is a key objective in developing biomimetic 3D functional architectures. For the aromatic oligoamide foldamer family, use of interactions between side-chains to control conformation is underexplored. The current manuscript addresses this objective through the design, synthesis and conformational analyses of model dimers derived from 3-O-alkylated para-aminobenzoic acid monomers. The O-alkyl groups on these foldamers are capable of adopting syn- or anti-conformers through rotation around the Ar–CO/NH axes. In the syn-conformation this allows the foldamer to act as a topographical mimic of the α-helix whereby the O-alkyl groups mimic the spatial orientation of the i and i + 4 side-chains from the α-helix. Using molecular modelling and 2D NMR analyses, this work illustrates that covalent links and hydrogen-bonding interactions between side-chains can bias the conformation in favour of the α-helix mimicking syn-conformer, offering insight that may be more widely applied to control secondary structure in foldamers.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...