Telescoped synthesis of C3-functionalized (E)-arylamidines using Ugi–Mumm and regiospecific quinazolinone rearrangements†
Abstract
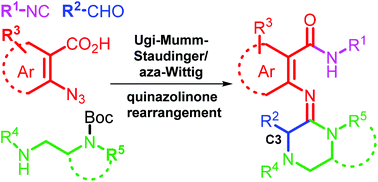
An efficient four-step, six-transformation protocol was developed to afford bioactive N-alkyl- or N-arylamide (E)-arylamidines featuring strategic amidine C3 modifications which were inaccessible or low yielding by previous methods. This synthetic approach, exemplified with 24 amidines and requiring only a single purification, highlights a multicomponent Ugi–Mumm rearrangement to afford highly diversified quinazolinones which undergo regiospecific rearrangement to afford new amidines. The method extensively broadens the structural scope of this new class of trisubstituted amidines and demonstrates the tolerance of regional C3 amidine steric bulk, visualized with X-ray crystallographic analysis.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...