α,β-Unsaturated butenolides in an organocatalytic doubly annulative cascade for the preparation of 3,4-dihydrocoumarins†
Abstract
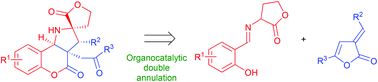
A new, organocatalytic doubly annulative cascade utilizing α,β-unsaturated butenolides and imines (derived from salicyl aldehydes and α-amino-γ-lactones) as starting materials is described. The developed strategy is based on two annulative processes: (1) initial [3 + 2]-dipolar cycloaddition allowing for the construction of a pyrrolidine ring; and (2) the butenolide-ring-opening reaction leading to the introduction of a 3,4-dihydrocoumarin framework. Target polycyclic compounds have been obtained with excellent chemical and stereochemical efficiencies.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...