N-Fluorobenzenesulfonimide as a highly effective Ag(i)-catalyst attenuator for tryptamine-derived ynesulfonamide cycloisomerization†
Abstract
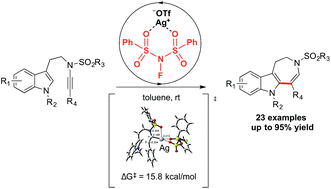
N-Fluorobenzenesulfonimide (NFSI) was identified for the first time as a highly effective Ag(I)-catalyst attenuator in the annulation of a tryptamine-derived ynesulfonamide to azepino[4,5-b]indole derivatives. Substrate tolerances were examined thoroughly and a plausible mechanism was proposed. The interaction between the Ag(I) catalyst and NFSI was probed by density functional theory (DFT) calculations. This study provided a highly efficient method to synthesize azepino[4,5-b]indole derivatives.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...