Photo-sensitized oxy-thiocyanation of terminal alkynes/1,3-aryldienes and their one-pot conversion to 2-hydroxy 4-substituted aryl thiazoles†‡
Abstract
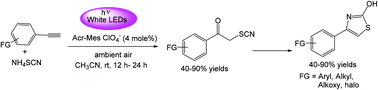
A regioselective visible light induced synthesis of aryl α-thiocyano ketones/thiocyano alcohols from activated terminal aryl alkynes and aryl 1,3-conjugated dienes was achieved. This mild and non-metallic oxidation is exclusively driven by benign ambient air in the presence of an organic photo-catalyst and NH4SCN. This protocol was also demonstrated at the 5 mmol scale for the synthesis of potentially therapeutic 2-hydroxy 4-substituted arylthiazoles in good yields, signifying its amenability for large-scale application.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...