Design, synthesis and study of a photochromic α,ω-diene: toward new classes of photoswitchable polymers†
Abstract
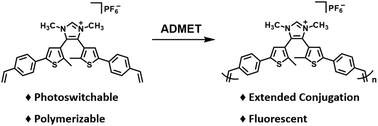
A 4,5-dithienylimidazolium salt outfitted with pendant styrenyl groups was synthesized and studied. The salt was found to undergo reversible electrocyclization upon UV irradiation; subsequent exposure to visible light reversed the reaction. Acyclic diene metathesis (ADMET) polymerization of the salt afforded a novel fluorescent polyelectrolyte.


 Please wait while we load your content...
Please wait while we load your content...