Catalytic asymmetric aza-Michael addition of fumaric monoacids with multifunctional thiourea/boronic acids†
Abstract
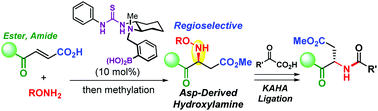
The first chemical enantioselective synthesis of N-hydroxyaspartic acid derivatives using chiral multifunctional thiourea/boronic acid organocatalysts was developed. A series of fumaric monoacids underwent an intermolecular asymmetric aza-Michael addition of O-alkyl hydroxylamines in excellent regioselectivity. The addition of another carboxylic acid raised the enantiomeric enrichment up to 97% ee. O-Deprotection of the aza-Michael adduct provided an aspartate-derived hydroxylamine fragment applicable for KAHA (α-keto acid-hydroxylamine) ligation.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...