In-flow photooxygenation of aminothienopyridinones generates iminopyridinedione PTP4A3 phosphatase inhibitors†
Abstract
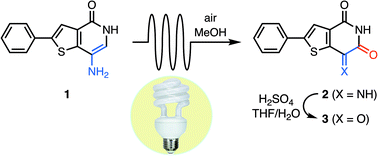
A continuous flow photooxygenation of 7-aminothieno[3,2-c]pyridin-4(5H)-ones to produce 7-iminothieno[3,2-c]pyridine-4,6(5H,7H)-diones has been developed, utilizing ambient air as the sole reactant. N-H Imines are formed as the major products, and excellent functional group tolerance and conversion on gram-scale without the need for chromatographic purification allow for facile late-stage diversification of the aminothienopyridinone scaffold. Several analogs exhibit potent in vitro inhibition of the cancer-associated protein tyrosine phosphatase PTP4A3, and the SAR supports an exploratory docking model.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...