Chemoselective syntheses of spirodihydrofuryl and spirocyclopropyl barbiturates via cascade reactions of barbiturate-based olefins and acetylacetone†
Abstract
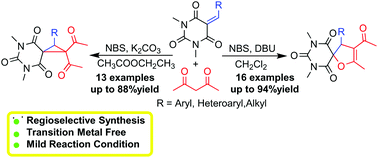
The Michael addition initiated ring closure reaction of barbiturate-based olefins and acetylacetone with NBS has been explored. The efficient and chemoselective approach for the synthesis of barbiturate-fused spirocycles was established. Spirodihydrofuryl barbiturates and spirocyclopropyl barbiturates were synthesized selectively via cascade reactions under different basic conditions in moderate to excellent yields. The structure of 2-(4-chlorophenyl)-1,1-diacetyl-5,7-dimethyl-5,7-diazaspiro[2,5]octane-4,6,8-trione was confirmed by single crystal X-ray diffraction analysis.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...