Total synthesis of (±)-galanthamine from GABA through regioselective aryne insertion†
Abstract
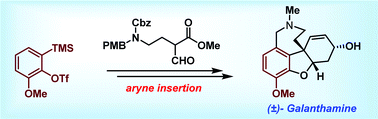
The total synthesis of (±)-galanthamine is achieved in ∼5% overall yield using a key regioselective aryne insertion reaction into a GABA (γ-amino butyric acid) derivative. The strategy presented involves only two sub-critical temperature reactions and less than five chromatographic purifications to achieve the synthesis of galanthamine.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...