Homopurine RP-stereodefined phosphorothioate analogs of DNA with hampered Watson–Crick base pairings form Hoogsteen paired parallel duplexes with (2′-OMe)-RNAs†
Abstract
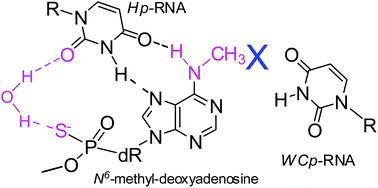
3′-O-(2-Thio-4,4-pentamethylene-1,3,2-oxathiaphospholane) derivatives of 5′-O-DMT-N6-methyl-deoxyadenosine and 5′-O-DMT-N2,N2-dimethyl-O6-diphenylcarbamoyl-deoxyguanosine (OTP-NY, NY = DMT-m6dA or DMT-m,m2dGDPC) were synthesized, resolved onto pure P-diastereomers, and used in P-stereocontrolled synthesis of dinucleoside 3′,5,-phosphorothioates NXPST (NX = m6dA or m,m2dG), in which the absolute configuration of the stereogenic phosphorus atom was established enzymatically. Diastereomerically pure OTP-NY and standard OTP-N (N = DMT-dABz or DMT-dGBz,DPC) were used in the synthesis of chimeric RP-stereodefined phosphorothioate oligomers ((RP-PS)-DN(NX)A) with hampered Watson–Crick base pairings. It was found that the m6dA units slightly reduce the thermodynamic stability of antiparallel duplexes formed with RNA and (2′-OMe)-RNA matrices, whereas m,m2dG units prevent their formation. The m6dA units stabilize (by up to 4.5 °C per modified unit) the parallel duplexes formed by (RP-PS)-DN(NX)A with Hoogsteen-paired (2′-OMe)-RNA templates compared to the analogous reference duplex containing only unmodified nucleobases. In contrast, the m,m2dG units destabilize such duplexes by up to 3 °C per modified unit. Both units prevent the formation of the corresponding parallel triplexes.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...