Rh-Catalyzed tandem C–C/C–N bond formation of quinoxalines with alkynes leading to heterocyclic ammonium salts†
Abstract
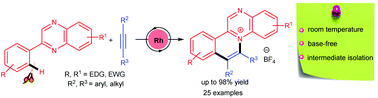
An efficient Rh-catalyzed oxidative C–H activation/annulation of 2-arylquinoxalines with internal alkynes is described using Cu(OAc)2·H2O and AgBF4 to afford a diverse variety of substituted quarternary ammonium salts at room temperature. The mechanism of the protocol is established on the basis of isolation of the 5-membered rhodacycle intermediate and kinetic isotope studies. The mild reaction conditions, substrate scope and functional group diversity are the salient practical features.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...