HFIP-promoted Michael reactions: direct para-selective C–H activation of anilines with maleimides†
Abstract
The Michael reaction is widely used for the C–C coupling of electron-poor olefins and C(sp3)–H pronucleophiles. Herein, an effective Michael reaction approach between electron-rich aromatic and heteroaromatic substrates as C(sp2)–H nucleophiles with maleimides as electrophiles in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) was first presented without the need for any additional metal catalysts or reagents. This reaction provides a concise and environmentally friendly strategy for the facile construction of 3-aryl succinimides from N,N-disubstituted anilines and maleimides with high para selectivity.
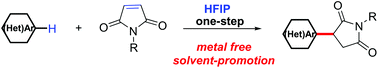
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...