Palladium-catalyzed oxidative amidation of quinoxalin-2(1H)-ones with acetonitrile: a highly efficient strategy toward 3-amidated quinoxalin-2(1H)-ones†
Abstract
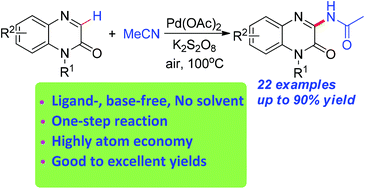
A novel and convenient palladium-catalyzed direct oxidative amidation of quinoxalin-2(1H)-ones with acetonitrile was developed to synthesize 3-amidated quinoxalin-2(1H)-ones. A series of 3-acetamino quinoxalin-2(1H)-one derivatives were constructed with good to excellent yields. This methodology provided a practical approach to various 3-acetamino quinoxalin-2(1H)-ones from the readily available starting material acetonitrile.


 Please wait while we load your content...
Please wait while we load your content...