Enantioselective synthesis of chiral heterocyclic biaryls via asymmetric Suzuki–Miyaura cross-coupling of 3-bromopyridine derivatives†
Abstract
A series of chiral heterocyclic biaryls with a pyridyl moiety were prepared in moderate to good yields with up to 92% ee via asymmetric Suzuki–Miyaura coupling. The chiral-bridged biphenyl monophosphine ligand L1 was found to be much more effective in the reaction enantioselection than its counterpart binaphthyl monophosphine ligands.
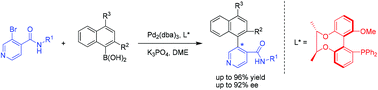
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...