Chorismatases – the family is growing†
Abstract
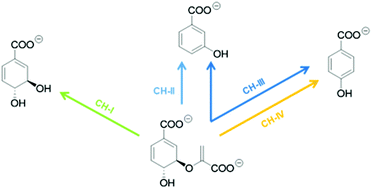
Chorismatases catalyse the cleavage of chorismate, yielding (dihydroxy-)benzoate derivatives, which often constitute starter units for pharmaceutically relevant secondary metabolites. Depending on their products, chorismatases have been classified into three different subfamilies. These can be assigned using a set of amino acid residues in the active site. Here, we describe five new chorismatases, two of them members of a new subfamily, which has been discovered through correlation analysis of homologous protein sequences. The enzymes from the new subfamily produce exclusively 4-hydroxybenzoate, the same compound as produced by the structurally unrelated chorismate lyases. This showcase of convergent evolution is an example of the existence of more than one pathway to central building blocks. In contrast to chorismate lyases, however, chorismatases do not suffer from product inhibition (up to 2 mM 4-HBA), while the remaining kinetic parameters are in the same range; this makes them an interesting alternative for biocatalytic applications.
- This article is part of the themed collection: Biosynthesis


 Please wait while we load your content...
Please wait while we load your content...