Stereoselective synthesis of a phosphonate pThr mimetic via palladium-catalyzed γ-C(sp3)–H activation for peptide preparation†
Abstract
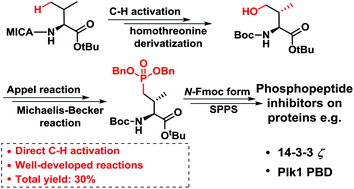
We report a facile synthetic strategy toward CH2-substituted phosphothreonine mimetics. Herein, inexpensive valine with a directing group was converted into homothreonine via palladium-catalyzed γ-methyl C(sp3)–H bond activation, followed by construction of a phosphorus–carbon bond via the well-developed Appel reaction and Michaelis–Becker reaction with a total yield of 30%. Furthermore, the derived mimetic was applied for solid-phase synthesis of two phosphopeptide inhibitors. This efficient synthesis provides a chance to prepare not only phosphopeptides but also phosphoproteins resistant to phosphatases.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...