An atom efficient synthesis of tamoxifen†
Abstract
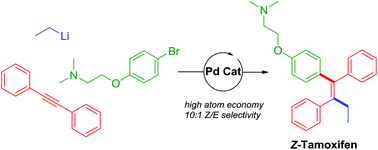
The direct carbolithiation of diphenylacetylenes and their cross-coupling procedure taking advantage of the intermediate alkenyllithium reagents are presented. By employing our recently discovered highly active palladium nanoparticle based catalyst, we were able to couple an alkenyllithium reagent with a high (Z/E) selectivity (10 : 1) and good yield to give the breast cancer drug tamoxifen in just 2 steps from commercially available starting materials and with excellent atom economy and reaction mass efficiency.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...