Cross 1,3-dipolar cycloadditions of C,N-cyclic azomethine imines with an N-benzyl azomethine ylide: facile access to fused tricyclic 1,2,4-hexahydrotriazines†
Abstract
A cross 1,3-dipolar cycloaddition of two different ylides between C,N-cyclic azomethine imines with an in situ-generated nonstabilized azomethine ylide from an N-benzyl precursor was realized. The reactions afforded a clean and facile access to diverse fused tricyclic 1,2,4-hexahydrotriazines in high yields (up to 96%). The chemical structures of the typical compounds were confirmed by X-ray single-crystal structure analysis.
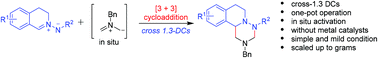
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...