A catalyst-free four-component domino reaction for the synthesis of functionalized 3-acyl-1,5-benzodiazepines†
Abstract
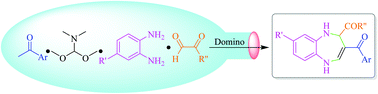
Various functional 3-acyl-1,5-benzodiazepines containing carboxyl, ester and acyl groups at the 2-position were synthesized via an efficient, sustainable and catalyst-free domino reaction. During the synthesis process, one new cycle and four new bonds (one C–C, two C–N and one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) were constructed by the nucleophilic substitution, nucleophilic addition, dehydration and cyclization reaction by the H+ shift. Furthermore, a total of 26 examples were examined by reacting inexpensive starting materials of N,N-dimethylformamide dimethyl acetal, aromatic ketones, 1,2-phenylenediamine compounds and aldehyde derivatives. Therefore, it displayed a broad substrate scope, good functional group tolerance, high yields (77–97%) and the ease of obtaining target compounds without the involvement of toxic solvents and column chromatography, which provided a novel method for the synthesis of a wide variety of biologically relevant 1,5-benzodiazepines.
C) were constructed by the nucleophilic substitution, nucleophilic addition, dehydration and cyclization reaction by the H+ shift. Furthermore, a total of 26 examples were examined by reacting inexpensive starting materials of N,N-dimethylformamide dimethyl acetal, aromatic ketones, 1,2-phenylenediamine compounds and aldehyde derivatives. Therefore, it displayed a broad substrate scope, good functional group tolerance, high yields (77–97%) and the ease of obtaining target compounds without the involvement of toxic solvents and column chromatography, which provided a novel method for the synthesis of a wide variety of biologically relevant 1,5-benzodiazepines.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...